Introduction
The magic of hot and cold packs lies in the chemical reactions inside; they undergo a dramatic temperature change after an activation step. You may have noticed in previous experiments that sometimes dissolving a salt makes a solution warm, other times it will make the solution cold. This property of salts is the basis for many commercial hot packs and cold packs. The activation step causes the salt and the liquid to mix, which either increases or decreases the temperature, depending on the salt. In this experiment, you will measure the heats of solution for several salts.
A reusable hot pack is a little different. It contains a supersaturated salt solution, and the activation step seeds the crystallization of the salt. Then heat is released when the salt crystallizes. It is reusable because you can heat up the solution in hot water or a microwave to dissolve the salt and reform the supersaturated solution. Today, you will also make a supersaturated solution and then measure the temperature change upon crystallization, similar to a reusable hot pack.
Background
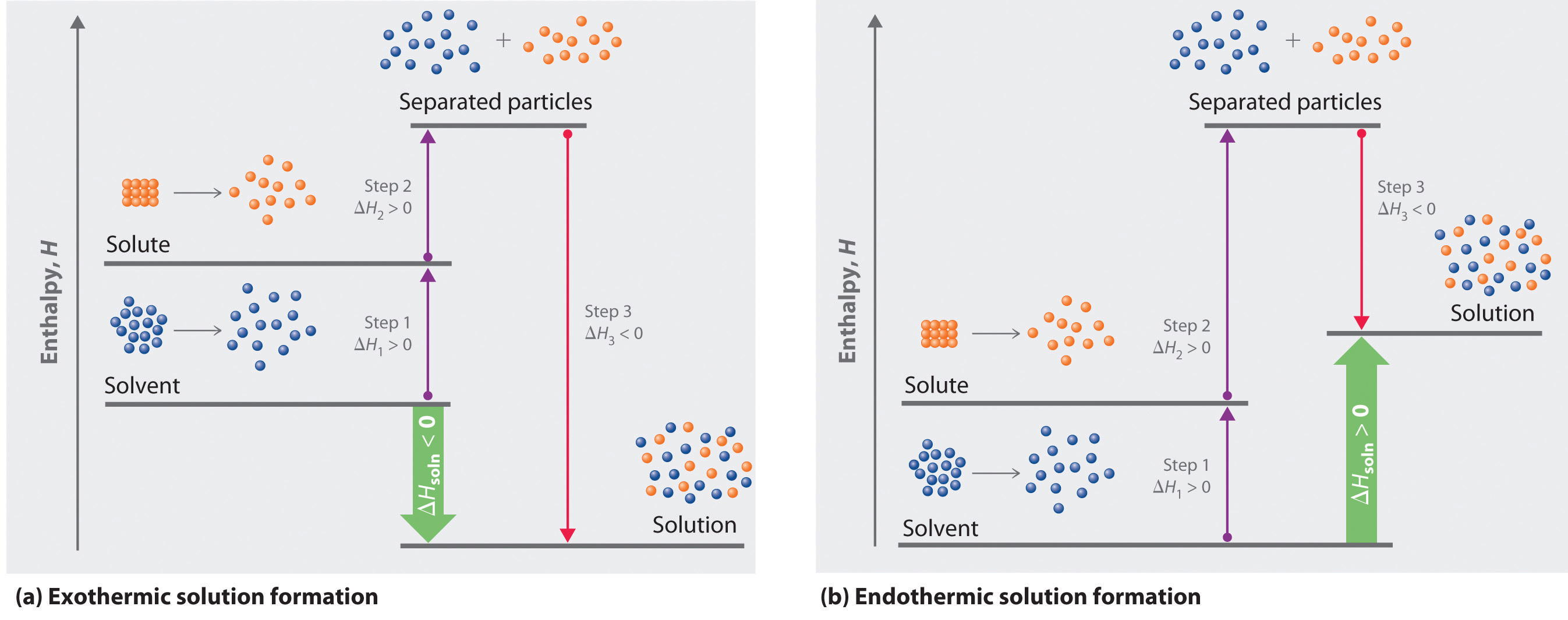
The ability of a salt to release or absorb energy upon solution is quantified as the enthalpy of solution. To measure the enthalpy of solution experimentally, we can use a solution calorimeter to measure a temperature change of a known mass:
q = mass * specific heat * ΔT
where:
mass = total mass of the combined salt and water in grams;
specific heat = specific heat of the solution. we will approximate it as the specific heat of water, which is 4.18 J/g oC;
ΔT = the difference between the starting temperature and final temperature in oC.
This gives you q for the water. Since the q for the water is the opposite of the q for the salt, you will need to switch the sign of q for water to get q for the salt. If you want to compare your answer to a literature value, you have to standardize q for the salt to ΔHo for the salt: convert J to kJ, then divide by the number of moles of salt you used, which gives you a value in kJ/mol. Note: this conversion is only valid under conditions of constant pressure.
This is a good web resource to learn more about enthalpy of solution.
http://images.flatworldknowledge.com/averillfwk/averillfwk-fig13_001.jpg
Similarly, the ability of a salt to release or absorb energy upon crystallization from a saturated solution is quantified as the heat of fusion. Experimentally, you can measure the heat of fusion by observing a temperature change when a salt is allowed to crystalize. The calculations all work essentially the same way and the final answer is a ΔH in kilojoules/mol.
Procedure
Note: do this experiment with a partner. You need two thermometers.
Part I: Prepare a supersaturated solution
- Thoroughly wash a 125 mL Erlenmeyer flask. Rinse with DIH2O. It does not need to be dry. Weigh the flask, and record the mass on Table 2.
- Measure out 50 grams of sodium acetate trihydrate directly into a tared 125 mL Erlenmeyer flask. Record the exact mass on Table 2.
- Add approximately 40 mL of DI H2O.
- In a fume hood with the sash lowered, heat the flask gently over a bunsen burner until it starts to boil and the crystals dissolve. Using the wash bottle filled with DIH2O, rinse all of the crystals down into the water, but try not to add too much water.
- Once the crystals are dissolved, remove the flask from the heat, put a thermometer into a split rubber stopper, lower the thermometer into the solution, stopper the top, and let it cool undisturbed. Note: If it refuses to dissolve even at boiling, then add another 1 or 2 mL of DIH2O.
Part II: Measure enthalpy of solution
To measure the enthalpy of solution, quickly add approximately 5 g of the salt to approximately 50 mL of temperature stabilized water. Put the lid in place and lower the thermometer into the solution. Swirl to dissolve while monitoring the temperature for at least 2 minutes. Be sure you are not clutching the thermometer too much with your hand, or your body heat will affect the reading. Record all temperatures to the nearest 0.2 oC. When measuring masses and/or volumes, choose appropriate tools to ensure that the temperature change limits the significant figures in the final answer. Pour the solution down the drain. Rinse and dry the calorimeter before performing another trial. Hint: you may want to attempt a calculation after your first trial to be sure you collected all of the data needed to complete your calculations. If time allows, discuss the results of your first trial with your instructor or TA for recommendations on improving the experiment before subsequent trials.
Part III: Measure the temperature change of crystallization
- Check the temperature of the supersaturated solution. If the temperature is above 30 oC, make a cold water bath and place the flask in the cold water bath for a few minutes to cool it to 30 oC.
- Without touching the thermometer, measure the temperature of the supersaturated solution. Record the temperature as the “initial temperature” on Table 2 to the nearest 0.2 oC.
- Obtain a small, single crystal of the sodium acetate salt. Carefully drop it into the flask.
- Observe the temperature change as the solution crystallizes. Record the highest temperature achieved as the “final temperature” on Table 2 to the nearest 0.2 oC.
- Remove the stopper and thermometer. Weigh the solution in the Erlenmeyer flask. Record the mass on Table 2.
- Use excess water to redissolve the salt and wash it down the drain.
Report
Use this resource to find literature values of heats of dissolution.
Complete the worksheet. Turn in either a paper or digital copy.






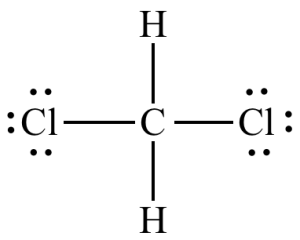

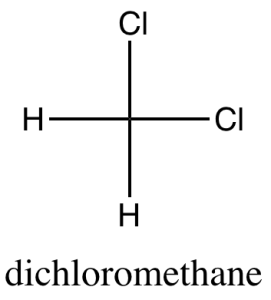 software. It calculates 3D structures and molecular orbitals, which help chemists predict the chemical and physical properties of theoretical molecules. In this lab, you will use ChemDraw to analyze the structures of molecules in 3D.
software. It calculates 3D structures and molecular orbitals, which help chemists predict the chemical and physical properties of theoretical molecules. In this lab, you will use ChemDraw to analyze the structures of molecules in 3D.