Learning Goals
- Employ several common separation techniques and analyze limitations of those techniques;
- Practice decantation and gravity filtration;
- Use a graduated pipette and an erlenmeyer flask;
- Observe a precipitation reaction and the formation of a crystalline solid;
- Use notions of stoichiometry to calculate reaction yield;
- Use a drying oven and analyze limitations of the drying oven.
Introduction

James Andrew Harris used separation chemistry to discover two new elements. http://www.cpnas.org/aahp/biographies/james-a-harris.html
James Andrew Harris was an American nuclear chemist who lived 1932 – 2000. While working at Lawrence Berkley National Laboratory (at the time, called Lawrence Radiation Laboratory), he helped discover synthetic elements 104 and 105, Rutherfordium and Dubnium. How are synthetic elements made? Often by smashing together very pure samples of lighter elements. Harris carefully produced these pure samples using separation chemistry.
These are a few more examples of the applications of separation chemistry today and in history:
- Isolating the fissionable Uranium and Plutonium isotopes that were used to make the atomic bombs dropped on Japan in World War II;
- Extracting metal from ore to make metal tools (ever heard of the “Iron Age” or “Bronze Age”?);
- Purifying drinking water;
- Purifying pharmaceuticals from natural sources;
- Distilling spirits;
- Treating sewage;
- Dialysis for patients suffering from kidney failure;
- Refining crude oil.
In this lab, you will be separating a mixture of substances using physical and chemical separation techniques and deducing the original masses of each substance in the mixture.
Background
The heterogeneous mixture you will begin with contains elemental iron filings, silicon dioxide (sand), sodium chloride, and sodium nitrate. You will be separating each of these substances using several common separation techniques that depend on the physical and chemical properties of the substances: magnetic separation, filtration, chemical coagulation, and finally evaporation.
- Magnetic separation uses a magnet to pull out magnetic particles (such as iron filings);
- Filtration removes insoluble particles (such as sand);
- Chemical coagulation is the introduction of a chemical that causes part of a mixture to precipitate out of a solution so it can be removed physically such as with filtration;
- Evaporation isolates soluble substances by evaporating water.
Procedure
Note: perform this lab with a partner.
I. Sample the mixture
- Weigh an 800 mL beaker. Record the mass in Table 2. The balance near the south entrance to the lab has the highest capacity. Use it if you have a heavy beaker.
- Pour in the contents of your jar. Tap or scrape out every little bit.
- Record the information written on the jar in Table 5 in the column labeled “original mass.” Table 5 is near the end of your lab worksheet.
II. Isolate the iron filings
- Record the mass of a 250 mL beaker in Table 1.
- Put a plastic bag over a bar magnet. Move the plastic-covered magnet around near the mixture. Observe the iron collecting on the magnet. Gently shake the magnet to keep sand and salt from collecting on the magnet: you only want iron filings to stick to the magnet.
- To remove the iron, put the magnet into the 250 mL beaker. Open the plastic bag and remove the magnet. The iron filings should drop off the bag into the beaker. If you find that salt and sand were also collected, then try again. Try performing this step a few times until you perfect your technique.
- Repeat the iron collection process three or four more times until no more iron accumulates on the magnet.
- Record the mass of the iron filings in the beaker in Table 1.
- After you have recorded the mass, put your recovered iron filings in the iron filings waste container.
III. Isolate the silicon dioxide
- Add about 100 mL of DI H2O into the 800 mL beaker that contains the mixture, now depleted of iron. Swirl rigorously for a few minutes, but take care not to splash any out of the beaker.
- Let the sand settle at the bottom of the beaker, then carefully pour the aqueous solution into a funnel in a 250 or 300 mL Erlenmeyer flask. Try not to lose any sand in the solution, but also try to pour off as much of the solution as possible. Imperfect separation in this step is a major source of error in this experiment. Record in your observations in Table 2, particularly note whether or not you lost sand in the solution and how wet your sand appeared after pouring off the aqueous solution (eg. “sand appeared soupy” or “sand had no visible liquid”).
- Label your beaker containing the sand with a sharpie directly on the glass. Put the beaker with the wet sand inside in the drying oven for 20-30 minutes. You should continue with the next part of the lab while this dries. Your instructor or TA will help you decide when to take the beaker out. When the sand flows freely, take it out and let the beaker cool completely before weighing the beaker with the sand inside and recording the mass in Table 2.
- Put the recovered sand into the trash.
IV. Precipitate chloride salt
- Weigh a piece of filter paper on a watch glass. Record the mass in Table 3.
- Use a graduated pipette to add 7 mL (notice the significant figures here?) of 3 M nitric acid [CAUTION!] to the solution. Swirl to mix.
- Use a graduated pipette to add 5.00 mL (notice the significant figures here?) of the prepared 0.500 M (and here?) silver nitrate solution to your solution in the Erlenmeyer flask. Observe a white precipitate forming upon mixing these two clear solutions. Swirl to mix. Pro-tip: Erlenmeyer flasks are a great choice when you want to swirl a solution to mix rather than using a stir rod, which may inadvertently remove product.
- Let the mixture settle in a dark cabinet for 5 minutes. Make sure to set up your filtration apparatus while you let it settle.
- Set up a filtration apparatus with the weighed piece of filter paper and small funnel, similar to the one in the illustration. Place the funnel into a 250 or 300 mL Erlenmeyer flask. Wet the filter paper with a little water to help it stick to the glass.
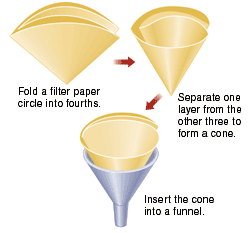
Filter apparatus https://alfa-img.com/show/filter-paper-and-funnel.html
- Once the mixture has settled for 5 minutes, pour the solution through the filter apparatus. If any solids stick to the flask, rinse the flask once or twice with a small amount of cool DI water. Note that any water you add will have to be boiled off, adding time to the end of the experiment! Scrape with the rubber policeman if necessary.
- After all of the solution has passed through, carefully move the filter paper to the watch glass without losing any of the solids. Unfold the paper. Label the watch glass. Put this into the drying oven for about 20 minutes until completely dry. Use this time wisely. You can clean up other parts of the experiment and/or continue the experiment. You don’t need to watch it dry. If it isn’t dry before you are ready to leave for the day, then take it out of the oven, put it in your cabinet, and come back another day to weigh it.
- Once dry, let the watch glass cool completely, then weigh it and record the mass on Table 3. Subtract the mass of the watch glass and filter paper to deduce the mass of the pure chloride salt. Dispose of the solids and filter paper in the trash.
V. Dry the nitrate salt
- Weigh your 800 mL beaker, and record the mass in Table 4 (yes, it might be different from the last time you weighed it. you might have mixed it up with someone else’s).
- Pour the solution now containing just the nitrate salt into the weighed 800 mL beaker.
- Set up a ring stand with wire gauze with a bunsen burner underneath, similar to the one in the illustration. Set this up in a fume hood.
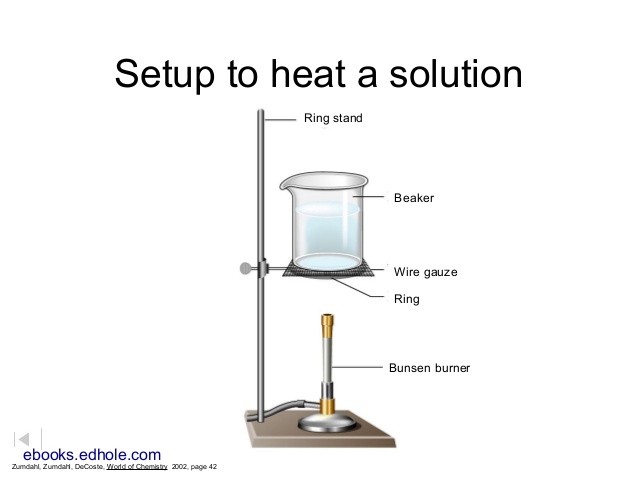
- With the hood sash closed, boil the solution until the volume decreases to about 10 mL or less. When the volume gets very low, watch it carefully for crystal formation. As soon as you see a crystal form, turn the gas down and stay close. When it is dry, turn the gas off. If you keep heating after it is dry, the salt will spatter (lost product) and the beaker may shatter (yikes!). After the beaker is cool, weigh the beaker and the crystals. Record the mass in Table 4. Wash the crystals down the drain. (Look at how soluble this product is!) Subtract the mass of the beaker to deduce the mass of the impure nitrate salt.
Calculations
The masses of the recovered iron filings and sand are directly measurable, but the masses of the salts require advanced stoichiometry to deduce. Embrace the challenge!
Report
Fill out this worksheet. Turn in either a paper or digital copy.
