The Crocker Lab is interested in understanding what happens at the molecular and cellular levels in the brain when an animal experiences a painful traumatic event. And in turn, how this changes behavior. We are interested in understanding why painful events are often our strongest memories and why they are so hard to forget. This work has relevance to our understanding of Post-Traumatic Stress Disorder (PTSD) and Traumatic Brain Injuries (TBI). We want to know what happens at a cellular level when the brain goes through a physically painful experience, either through exposure to electric shock or severe mechanical pain. In order to study how this changes the molecular and cellular properties of neurons, as well as behavior, we use the fruit fly (Drosophila melanogaster) as a model organism. The fruit fly has a rich history in genetics with a number of Nobel prizes awarded for work using it to understand development, and most recently circadian rhythms.
Below is a summary of ongoing projects in the lab.
Why is Electric Shock Painful?
Following up from previous work, we found a mutant fly that failed to avoid electric shock. This was fascinating to us and sent us down a path of trying to understand how electric shock is detected and processed in the brain. Surprisingly, very little is known about this across any organism.
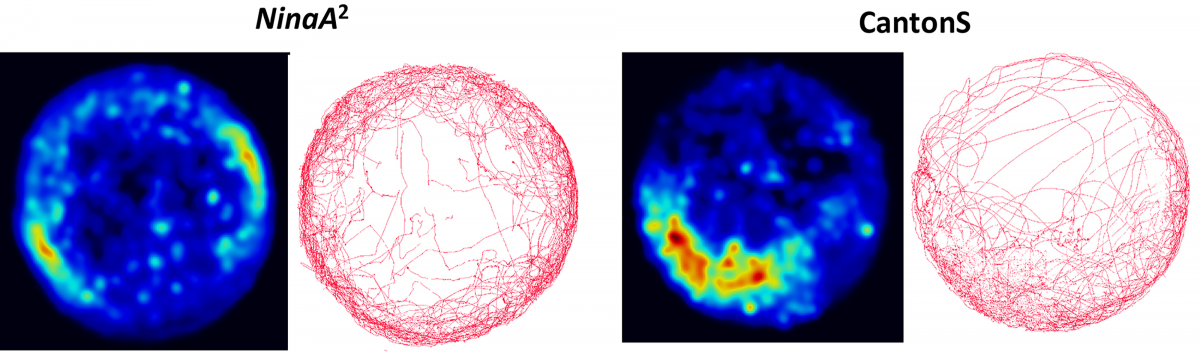
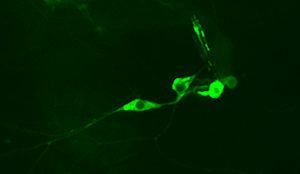
We have begun mapping in the fly larvae how electric shock is processed in the sensory neurons of the body wall. The fly provides unique set of tools to allow us to explore what electric shock is doing at a behavioral level (High resolution video), transcriptional level (RNAseq), translational and protein level (Ribotag and proteomics), and at a cellular activity level (Calcium imaging tools).
Traumatic Brain Injury
The use of fruit fly to model traumatic brain injury has exploded in the recent years. In the lab we use the “HIT” device to explore how a TBI changes an animals behavioral response to heat stress and social interaction. We are doing this across a large inbred population of Drosophila to explore the genes that play a role in these behaviors. We are also looking at the molecular aspects of TBI and mitochondrial function (or dysfunction).
Transcriptional and Mitochondrial Gene Regulation in Rett Syndrome
We have recently started collaborating with Dr. Victor Faundez at Emory University. Through this collaboration we are bring cutting edge sequencing and bioinformatics projects to Middlebury students. The data sets we are analyzing are important for our understanding of the roles certain genes and mutations play in neurological disorders. They provide insights into how the transcriptional and proteomic landscape shapes complex neurological disorders such as Rett’s syndrome.