adapted by M. J. Simpson from a lab originally by S.F. Sontum, S. Walstrum, and K. Jewett
Introduction
Theories of chemical bonding allow us to understand the electronic structure and geometrical arrangement of atoms in a molecule or ion. Models provide a useful way of visualizing the arrangement of electrons in a molecule. As you learned in class, there are several types of structure representations used by the chemist at different times to explain chemical phenomena. These include Lewis structures (including formal charges) based on a simple counting rule; valence bond models (including hybridization, and resonance) based on orbital overlap; valence shell electron pair repulsion theory (VESPR theory) based on electron repulsion, to predict overall shapes of molecules/ions; and molecular orbits to predict certain electrical and magnetic properties.
The figure above is a ball and stick model for an early precursor to one of the anti-HIV drugs known as Protease inhibitors developed at Abbott Laboratories, Inc. We need models like this because actual molecules are too small to see. Modeling of molecular structures allows us to make predictions about the behavior of these invisible molecules so that we can design new chemicals with beneficial properties such as these inhibitors. The ball and stick model kits you use in this chemistry laboratory are very useful for visualizing chemical structures but they do not give quantitative information needed to design and build new molecules with specific properties. ChemDraw is a computer “modeling kit” which we use to augment our visualization of the molecule with quantitative information.
Procedure
To illustrate how molecular geometry can be obtained from Lewis structures and valence shell electron pair repulsion, we will use molecular models. With models, it is relatively easy to see both geometry and polarity, as well as to deduce Lewis structures. You may want to initially generate your Lewis structures before you come to the laboratory. When you come to the laboratory use the molecular models to check and refine your Lewis structures. In this exercise you will assemble models for a number of common chemicals and interpret them in the ways we have discussed.
The models consist of plastic bonding centers and bonding tubes. The bonding centers represent the hybrid valence electron distribution about the atom. The tubes represent the bonding electron pairs or valence bonds. As you build the models also draw the three dimensional structures on paper, so that you can develop skills at representing these three dimensional structures on paper.
Most of the structures will obey the octet rule. Often the central atom will have four electron pairs and will have a tetrahedral bonding center. In your model kits, multiple bonds are modeled by the long, flexible bonds. We will see from our molecular orbital studies that the bent “banana” p bonds of our model kit are not good representations of the electron distribution but never the less correctly represent the Lewis structure.
Methane: Construct a model of methane, CH4.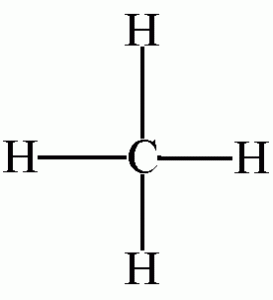
Place the model on the desk top and note the symmetry of this tetrahedral molecule. The molecule looks the same regardless of which three hydrogens are resting on the desk. All four hydrogens are said to be equivalent. Is this molecule polar? Draw a three dimensional picture of this molecule.
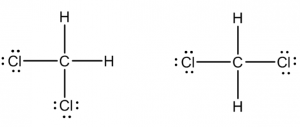
Dichloromethane: Replace two of the hydrogen atoms in methane with a chlorine to make dichloromethane CH2Cl2. Is dichloromethane polar? Which Lewis structure best represents dichloromethane or are they the same?
Ethyl Alcohol and Dimethyl Ether: Construct ethyl alcohol, CH3CH2OH, and dimethyl ether, CH3OCH3. The following is a chart of the physical properties of these two isomers.
| Boiling Point | Melting Point | Solubility in water | |
| Isomer 1 | 78.5 oC | -117 oC | Infinite |
| Isomer 2 | -24.0 oC | -139 oC | Slight |
Which isomer is more soluble in water? Which would have a higher boiling point? Why?
Constructing Lewis Structures
Now that you have some experience building molecular models from a Lewis structure you should be ready to fill in a geometrical structures chart starting only from the molecular formula. In assembling a molecular model of the kind we are considering, it is possible, indeed desirable, to proceed in a systematic manner. We will illustrate the recommended procedure by developing a model for the first molecule on the chart CH2O. Please work along with your model as we describe the procedure.
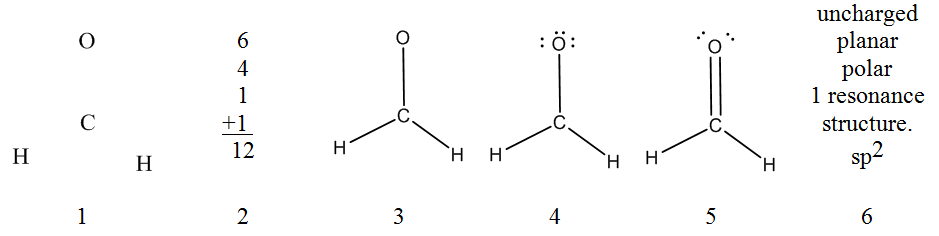
- Choose a skeleton with the least electronegative atom for the central atom.
For CH2O, this is carbon. Remember, ring structures may be possible with six member rings being the most stable and 3 member rings the least stable. - Determine the total number of valence electrons.
For CH2O this total would be 12 valence electrons. Now collect 6 bonding links representing the 6 pairs of bonding electrons. - Assemble a single bonded skeleton structure.
In CH2O the model can be assembled by using two links to form s bonds between C and H, and then using a third link to form a s bond between C and O. - Add lone pairs.
Use the remaining tubes to fill all the unfilled sites on the bonding center and to satisfy the octet rule. In the model, three links may be used to fill the O site leaving one unfilled sites on C. - Form multiple bonds as needed to satisfy the octet rule.
Move one of the lone pair electrons off of the oxygen to generate a double bond with carbon and satisfy the octet rule on carbon. - Interpretation of the Lewis Structure.
The tubes and spatial arrangement of the bonding centers will closely correspond to the electronic and atomic arrangement in the molecule. Be sure to check that you have a valid Lewis structure (octet rule) and the right total number of electrons. The Lewis structure of the molecule is given below: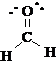
Formal Charge: Since all of the octet atoms satisfy the 8n rule there is no formal charge.
Hybridization: The angle between VSEPR groups is 120° so the hybridization is sp2 on both the carbon and the oxygen.
Resonance structures: The formation of other resonance structures would entail the moving of p bonds or the formation of new p bonds from lone pairs. For this molecule, there are no other ways to rearrange the electrons over the molecular skeleton and still satisfy the octet rule. This is the only possible full octet resonance structure for this molecular skeleton.
Atom Geometry: Given our model, we would describe the CH2O molecule as being planar with single bonds between carbon and hydrogen atoms and a double bond between the C and O atoms.
Polarity: Since all bonds are polar and the molecular symmetry does not cancel the polarity in CH2O, the molecule is polar. (The compound having molecules with the formulas CH2O is well known and is called formaldehyde. The bonding and structure of CH2O are correctly given by this model.)
Isomerism: A possible alternate skeleton and Lewis structure would be H–C=O–H but this structure results in a formally positive oxygen next to a negative carbon. Because of the unfavorable charge separation this molecule does not exist.
Introduction to ChemDraw
ChemDraw is another “modeling kit” which we use to augment our information about molecules, visualize the molecule, and give us quantitative information about the structure of molecules.
The following is a brief introduction on how to use ChemDraw.
- Open Chem3DPro
- Click on the white panel on the right labeled ChemBioDraw – LiveLink
- Make sure the Text tool is selected when the Tools menu pops up
- Type the formula. For example, SO32- is entered as SO3-2
- If you want a different isomer, you can enter the formula more specifically. For example, instead of C3H4, you might enter H2CCCH2
- Hit enter. The molecule will appear. If it is outlined in red, that means something is wrong with your molecule. Consult the lab instructor or TA if you cannot figure out the problem
- Hit control-M to minimize energy. The total energy will be displayed at the bottom under Output
- To measure bond lengths, hover the mouse over the bond
- To explore the structure in different modes, go to View → Model Display → Display Mode, and then pick another mode. Try a few different ones
- To show or hide lone pairs, go to Structure → Lone Pairs, and make your selection
- To save a structure as an image, go to File → Save As, and then change the file format to whatever you like (such as .png or .jpg)
- Hit control-N to start over
- Another way to build a molecule is to build a backbone with the solid bond building tool and then change the atom types manually. After building the backbone, just click on the intersection you want to change and type the symbol of the atom you want to change. Double click the bond to make a double bond. This is especially useful for building the P4 isomers
Report: Fill out the worksheet for the report.
