Research Program of Professor Jeffrey Byers
My program, undertaken entirely with Middlebury students involves two separate areas of synthetic organic chemistry: The invention of new types of molecular wires, and the invention of new radical-based reactions of value to synthetic and polymer chemists.
Through a variety of other internal and external funding sources, I typically had3-4 students working side-by-side with me each summer, with a comparable number during the academic year. Since beginning my career at Middlebury in 1986, I have been awarded one grant from The Research Corporation, two ACS/PRF grants, five NSF-RUI grants, and served as PI or co-PI on NSF instrumentation grants leading to the acquisition of an NMR, GC/MS, polarimeter, and LC/MS for use in research and course based-activities. With these grants, I have been able to involve over 100 undergraduate students in my research efforts. Among these students, over 30 went on to earn advanced degrees (mostly PhD’s) in chemistry, biochemistry, or related fields, or are currently enrolled and progressing towards this degree. A total of 34 of my former students have gone on to careers as physicians, with many of them gravitating towards research-based careers, and 4 have earned law degrees, and have established careers in patent law, putting the technical training which they received in the course of their research to use in a different venue.
Much of this work has culminated in publications and presentations at scientific meetings with Middlebury students as coauthors.
- Molecular Wire Project.
Molecular wires have been of interest to organic chemists and polymer chemists for many years. The ability to create conducting or semiconducting organic polymers, the thickness of one molecule (hence the term molecular wires) would constitute the ultimate in miniature electronics for high value devices. In fact, the first molecular wire, polyacetylene, was the basis for the 2000 Nobel prize in chemistry.

One of the noteworthy characteristics of polyacetylene, characteristic of all organic conductors, is the continued pi-bond resonance along the polymer chain. While polyacetylene opened up the field, the polymer in its neutral state is actually a rather poor conductor. Polyacetylene, and most other pure organic polymers, are only electronically useful through the process known as “doping”, in which a few electrons are added or removed from the conjugated system. Unfortunately, this also destabilizes the molecule, rendering it far less useful as a material. This drawback is why molecular wires have not seen any significant applications yet.
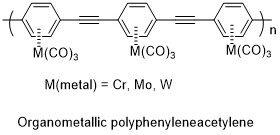
In the Byers group, we hope to synthesize organometallic polymers based on molecules with fully conjugated backbones. Many organometallic complexes have been made with metal-arene bonds. In bonds of this type, the metal (most commonly Cr, Mo, or W) is bound to the center of the benzene, effectively using all three pi bonds as Lewis Bases in the organometallic. While benzene itself is colorless, these organometallics are brilliantly colored (typically yellow or orange), the result of charge transfer between the metal and organic. It is our hope that this charge transfer process will result in a “self doping”, allowing for charge to pass more freely in a stable polymer consisting of these organmetallics linked through acetylenes. This approach also has the added potential benefit, of ligand substitution for CO, or additional substitution on the benzene rings as a means for modifying the properties of the molecule for stability or applications.
- Radical Reactions:
At the beginning of my career, I and my students discovered a new reaction which became known as a “Phenyl Selenide Radical Addition”. In many ways, this was a dream reaction to a synthetic organic chemist; It simply involved adding an organic molecule with a phenylseleno substituent to an alkene, dissolving it in benzene, and irradiating it with an inexpensive light source, an incandescent UV lamp (formerly available at low price in most hardware stores!) overnight. The products obtained by this reaction were astonishingly clean, with the addition product the only product formed in most cases. This reaction produced a new carbon-carbon bond, and introduced a phenylseleno group into the new molecule. While organoselenium chemistry is not a traditional part of undergraduate organic chemistry, there is a rich literature of transformations which can be accomplished with this functionality, putting it to further use in synthesis.

While this reaction, discovered at Middlebury, saw significant use for over 10 years in research groups around the world, its popularity declined as inexpensive sunlamps became unavailable, at least in the US. Curiously, so many people were giving themselves skin cancer with these primitive sources of UV light, that they were banned from sale! Unfortunately, the lack of availability of these lamps led to the diminished interest in this reaction, as other UV sources were either ineffective, or prohibitively expensive.
The recent ready availability of inexpensive and safe near-UV LED lamps has led us to re-evaluate this reaction. We hope to see if these new light sources will work as well as the old sunlamps, and revitalize interest in this radical addition reaction. While simple photolysis should prove ideal, we will also examine other newer techniques such as photoredox catalysis and Cu(I) catalysis, in the hope that this 20th century reaction can find new relevance in the 21st century.
We are also interested in new applications of this reaction. An important new class of reactions are generally referred to as “click” reactions. Click reactions are means of building up molecules with a family of reactions which work reproducibly, in high yield, under innocuous reaction conditions and in high yield. These click reactions are one of the most important families of synthetic tools for practitioners of nanotechnology. Simply put, they are used to “click” molecules together. Given the past ease with which these reactions have been carried out, we are re-examining our chemistry under new conditions for this purpose. Finally, we are trying to determine if these new techniques allow us to substitute Sulfur (S) for Selenium (Se). While organosulfur compounds are generally less reactive than their organoselenium counterparts, and failed to react under sunlamp photolysis, they would have the benefit of much lower cost, and in some cases, lower toxicity if contemporary conditions would render them useful.