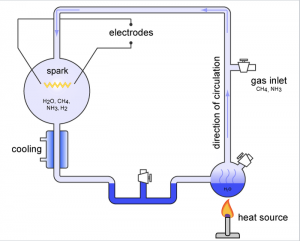
The Miller–Urey experiment was intended to simulate the conditions thought at the time to be present on the early Earth in order to test the chemical origin of life. It was done in 1952 by Stanley Miller, at the University of Chicago, but eventually Harold Urey, from the University of California, San Diego helped. “The experiment used water (H2O), methane (CH4), ammonia (NH3), and hydrogen (H2). The chemicals were all sealed inside a sterile 5-liter glass flask connected to a 500 ml flask half-full of liquid water. The liquid water in the smaller flask was heated to induce evaporation, and the water vapour was allowed to enter the larger flask. Continuous electrical sparks were fired between the electrodes to simulate lightning in the water vapour and gaseous mixture, and then the simulated atmosphere was cooled again so that the water condensed and trickled into a U-shaped trap at the bottom of the apparatus.” The two scientist concluded that according to their experiment life could have been naturally formed and since then the experiment is considered the classical scientific example of abiogenesis.

You must be logged in to post a comment.