[bg_faq_start]
Preparing chemical structures and reactions
Chemists often need to include drawings of chemical structures or reactions in their papers, such as when a new structure has been synthesized or a new mechanism elucidated. As with any figure or diagram, your goal in preparing these structures should be to get information across clearly. This means that authors need to label important structures, keep lines neat, and organize information sensibly. Drawing software such as Chem Draw (available from Middlebury College here) is highly recommended for preparing neat and accurate structures.
Every journal will have its own requirements for preparing and submitting structures. For more general reference, here we summarize the guidelines provided by the ACS Style Guide for drawing and preparing structures, reactions, schemes, and charts.
| ACS guidelines for preparing chemical structures |
|
| Make the size of structures and the size of the corresponding text proportional. For example, a 1/4-inch diameter ring size should have text of about 5 – 8 points. |  Image adapted from Armstrong et al. (2005) Image adapted from Armstrong et al. (2005) |
| Center compound labels just below their structures. |  Image adapted from Armstrong et al. (2005) Image adapted from Armstrong et al. (2005) |
| If discussing a group of related structures, draw only one parent structure. Use an “R” or similar designation to mark where the substituents differ and note modifications below the structure. | 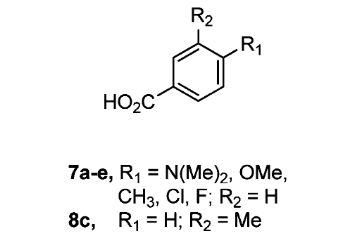 Image adapted from Armstrong et al. (2012) Image adapted from Armstrong et al. (2012) |
| In large or multiring structures, use partial structures and show only the pertinent portions. |  Image adapted from Armstrong et al. (2005) Image adapted from Armstrong et al. (2005) |
| Align the centers of all structures in the same row and arrange reaction arrows on this center line. Give arrows and plus signs equal amounts of room on both sides. |  Image adapted from the ACS Style Guide Image adapted from the ACS Style Guide |
Furthermore, ACS and the Journal of Organic Chemistry specify that drawings should adhere as close as possible to the following settings:
| Chemical structure drawing settings |
|
| chain angle | 120° |
| bond spacing | 18% of length |
| fixed length | 14.4 pt (0.508 cm, 0.2 in.) |
| bold width | 2.0 pt (0.071 cm, 0.0278 in.) |
| line width | 0.6 pt (0.021 cm, 0.0084 in.) |
| margin width | 1.6 pt (0.056 cm, 0.0222 in.) |
| hash spacing | 2.5 pt (0.088 cm, 0.0347 in.) |
| font | Arial or Helvetica |
| size | 10 pt |
Whether or not you choose to adhere to ACS’s strict standards or not is probably up to you for the purposes of most classwork. Remember that most important is that your structures are clear, neat, and easy to follow.
[bg_faq_end]
[bg_faq_start]
Formatting citations and references
To help you cite and reference material in your papers, we are providing some of the most common citation styles used in chemistry. More important than following any one style, however, is being consistent within a paper or poster. Make sure all of your citations and references are formatted the same way so that readers can easily find information about the sources of your information.
In-text citation styles
Most ACS journals use citation style 1 (superscript numbers). Most other chemistry journals will follow these same guidelines below, others with minor modifications. You can always check the guidelines for authors provided by a particular journal for specific requirements of a style you want to use. This information is often available via a link on the home page of a publication’s website.
- Superscript numbers
- Place citations after commas or periods
- Use commas (with no spaces) between citation numbers
Example: “Allose has been found to inhibit growth of human ovarian,5 head and neck,12 cervical,2 and multiple prostate8,13 and liver12 cancer cell lines in vitro.”
- Numbers in parentheses
- Place citations before commas or periods
- Italicize citation numbers and place them in parentheses ( )
- Place a space between the text and the brackets or parentheses
- Use commas (with spaces) between numbers
Example: “High concentrations of molecular gas have been identified in ultraluminous galaxies (4, 6-8, 12).”
Reference styles
The ACS recommends using one of the following two formats:
- Author 1; Author 2; Author 3; etc. Title of Article. Journal Abbreviation Year, Volume, Inclusive Pagination.
- Author 1; Author 2; Author 3; etc. Journal Abbreviation Year, Volume, Inclusive Pagination.
Notes: Some publications list only the first 10 authors followed by a semicolon and “et al.” Also, when abbreviating journal titles, use the Chemical Abstracts Source Index (CASSI) found here. Because the journal portion of a reference does not end in a period, only abbreviated words will have periods after them.
We highly recommend using citation software such as Zotero, which will create correctly-formatted citations and references for you! It takes a little while to get used to electronically storing citation information but will ultimately save you lots of time and hassle.
For more information, visit our pages on formatting citations and references as well as our page on using the literature in general.
[bg_faq_end]
[bg_faq_start]
Writing the experimental section of an organic paper
When a chemist synthesizes a new compound, it is important that they fully characterize it with relevant physical and spectroscopic data. Journals will have specific requirements for characterizing different types of molecules, but often data from IR spectroscopy, NMR spectroscopy, mass spectrometry, electronic spectroscopy, electron microscopy (TEM and SEM) and/or elemental analysis might be needed. In order to list this type of information clearly and concisely, it is common practice in many journals to list these type of data for each compound in separate sub-sections of the experimental section.
For instance, the Journal of Fluorine Chemistry (2014) requires the following information in the order given:
|
Furthermore, organic chemists tend to adopt a reversed sentence structure, describing procedures using a “to X was added Y” (rather than a “Y was added to X”) format.
To a cooled (ice-salt) suspension of AlCl3 (3.86 g, 28.9 mmol) in 1,2-dichloroethane (DCE; 50 mL) was added a solution of acetyl chloride (2.48 g, 31.6 mmol) in DCE (4 mL). (Adapted from Yasunaga et al. 1997)
This structure is used primarily to introduce the main subject of the sentence, such as the suspension, before the additive.
|
Adapted from Trabelsi, Szönyi, and Geribaldi (2001)
4. Experimental section 4.1 General The starting 2-perfluorobutylethyl-α-bromoacetate [26, 27] is prepared following standard literature procedures. THF was distilled over NAH immediately prior to use. All reactions were conducted under an atmosphere of dry N2. For 1H NMR, TMS as an internal standard. For 19F NMR, CFCl3 as an internal standard. The solvent for NMR measurement was CDCl3. 4.2 Alkylation of dialkyl malonate with 2-perfluoroalkyl-1-iodo ethane in presence of NaH To a suspension of 50 × 10-3 mol of NaH (80% in oil) in dry 100 ml of THF, 0.1 mol of dialkyl malonate was added dropwise over 30 min. To this solution, 50 × 10-3 mol of 2-perfluoroalkyl-1-iodo ethane was added dropwise, and the mixture was refluxed for 18 h. The solvent was evaporated and the residue dissolved in 100 ml of Et2O. The organic phase was washed with 30 ml of H2O and dried (Na2SO4). The Et2O layer was concentrated under reduced pressure. The resulting oil was purified by distillation under reduced pressure to yield products 4a–f. 4a (85%), Colorless oil, bp 65° C/0.2 Torr—IR (ν cm-1): 1753 (C=O), 1738 (C=O), 1300-1100 (CF) — 1H NMR (δ ppm; J Hz): 1.3 (t, 6H, 2 × OCH2CH3; J = 7), 2.21 (m, 4H, CH2CH2C4F9), 3.45 (t, 1H, CH; J = 7), 4.3 (q, 4H, 2 × OCH2CH3; J = 7) — 19F NMR (δ ppm): 81.5 (3F, CF3), 115.3 (2F, CF2), 124.8 (2F, CF2), 126.5 (2F, CF2) — MS (m/z, species, %): 406, M+, 2; 55, peak of base, 100 — Anal. C13H15F9O4 (406.2): calcd. C, 38.44; H, 3.72; F, 42.09; found C, 38.56; H, 3.63; F, 42.29%. 4b (85%) … |
[bg_faq_end]
[bg_faq_start]
Referring to compounds and reactions from schemes
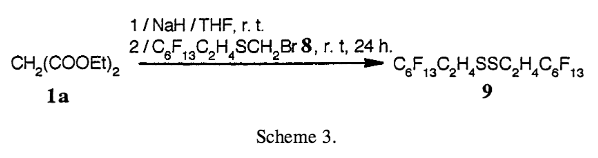
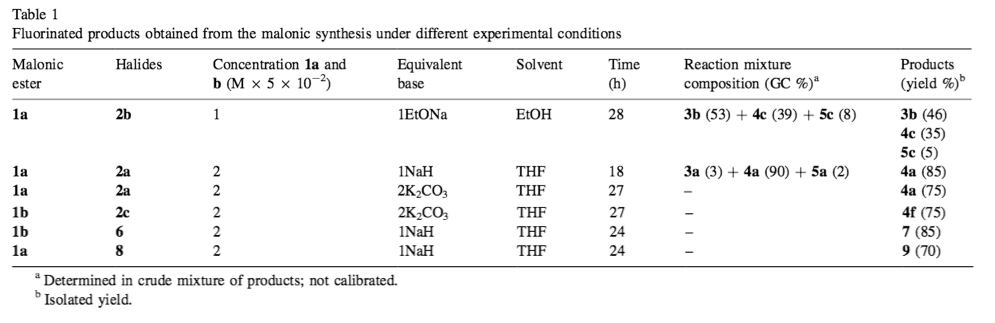
In synthesis or related papers, authors often have to refer to different compounds numbering on the order of tens. The convention used to keep all the different compounds straight is to identify each compound you reference with a boldface letter, number, or combination of letters and numbers.
| Some acceptable labeling series for compounds | |
| 1, 2, 3 … | A, B, C … |
| I, II, III … | a, b, c … |
| IA, IB, IC … | 1a, 1b, 1c … |
Use whichever labeling series works best for the content in your paper, but make sure to use them consistently within the paper. Label every structure in a scheme, as long as the structure is also discussed in the text. The first time you refer to a specific compound in the text, define it, such as:
- “to the mixture was added one equivalent of 2-perfluorohexyl-1-iodoethane (2b)” OR
- “to the mixture was added one equivalent of 2-perfluorohexyl-1-iodoethane 2b.”
In subsequent mentions of the same structure, you can refer to it using a general descriptor followed by its label or only by its label, such as:
- “IR identified ethylene compound 2b in the solution”
- “IR identified 2b in the solution” or
- in a figure or table
This type of labeling system should also be used for the chemical reactions in which the structures are found. Reaction reference labels should be separate from compound label sequences and be in lightface, not boldface. Depending on your paper, it may be appropriate to use one labeling sequence for both mathematical and chemical equations, or you may want to keep them separate.
| Examples adapted from Trabelsi, Szönyi, and Geribaldi (2001) |
|
2. Results and Discussion … Adding an excess of potassium carbonate and an excess of the starting active methylene compound to the reaction mixture allows complete conversion of the halide. Thus, although the reaction times are long, better results were obtained for mono-alkylated compounds, and neither elimination nor dialkylation is observed (see Scheme 1 and Table 1). … The present malonic ester synthesis of polyfluoroakyl malonic esters compounds may be of more general use.
|
[bg_faq_end]