Learning goals: maintain safety in a chemistry laboratory, follow instructions to complete a laboratory experiment, collect experimental data, explain likely sources of experimental error
Introduction
A chemical reaction occurs when electrons and/or bonds rearrange. A chemical reaction can be described with a chemical equation. You may notice a chemical reaction has occurred by one of the following indicators:
- A color change (eg. a colorless solution turns red),
- A change of state (eg. a solid precipitates in a solution),
- A change in temperature (eg. a solution gets hot).
Today’s lab activity is an example of a chemical reaction. You will form a golden solid from two colorless solids.
Background
Lead nitrate and potassium iodide are both highly soluble in water. This means:
Pb(NO3)2 (s) → Pb2+(aq) + 2NO3–(aq)
KI(s) → K+(aq) + I–(aq)
When you mix those two solutions together, all four ions encounter each other, and that’s when the chemical reaction occurs:
Pb2+(aq) + 2I–(aq) → PbI2(s)
The potassium and nitrate ions are not involved in the chemical reaction; they are called “spectator ions.” They are present in the same amounts before and after the reaction (on both sides of the arrow).
Pb2+(aq) + 2NO3–(aq) + 2K+(aq) + 2I–(aq) → PbI2(s) + 2NO3–(aq) + 2K+(aq)
When the solid forms, it first looks like yellow clouds because the tiny solid particles are suspended in the solution (like mud). We have to take advantage of its slight solubility in order to form a sparkling, golden solid. When you first add the lead nitrate to the potassium iodide, you will notice the cloudy precipitate forms and then dissolves. This is because lead nitrate is “slightly soluble.” 0.08 g of it can dissolve in 100 mL of water: the yellow clouds only remain visible when there is more than 80 mg present per 100 mL of water.
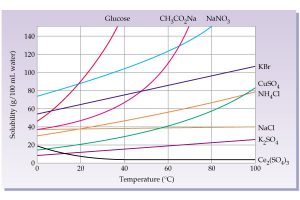
Temperature dependence of solubility for several solids. Source: http://wps.prenhall.com
Many solids are more soluble in hot water than cold. Heating the solution will cause the lead nitrate to dissolve completely so that it can slowly crystallize as it cools, which forms large crystals.
Procedure
Note: do this lab individually.
Prepare solutions
- Tare a 250 or 300 mL Erlenmeyer flask labeled “lead nitrate”. Add approximately 0.3 g of lead nitrate to the flask. Record the exact mass on the worksheet.
- Measure out 100 mL of deionized water (DI H2O) with a graduated cylinder. Pour the water into the flask containing the solid lead nitrate. Swirl to dissolve.
- Repeat this procedure with another flask for the Potassium Iodide.
- Add a few drops of 0.5 M hydrochloric acid to each solution.
Form Crystalline Lead Iodide
- Add the lead nitrate to the potassium iodide one drop at a time, swirling to dissolve after each drop. How many drops can dissolve? Once the yellow clouds start to form and will not dissolve when swirling, pour in the remainder of the lead nitrate. Record your observations.
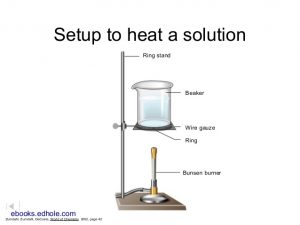
- Prepare a hot water bath with a Bunsen burner, a ring stand, a ring, a wire mesh, and an 800 mL or 1000 mL beaker – similar to the picture to the right. Alternatively, you can use a tripod instead of the ring stand and ring. Put approximately 500 mL of water in the beaker, then heat the water up to boiling. (Hint: If you read through these instructions before coming to lab, you will probably start with this step so you do not have to wait for your water to heat up.)
- Carefully place the Erlenmeyer flask containing the lead iodide in the hot water bath. Heat the lead iodide solution up to about 80oC or until the lead iodide dissolves. Record your observations.
- Once the lead iodide has completely dissolved, remove the Erlenmeyer flask from the hot water bath. Set it on the bench top and watch the crystals form. Wait about half an hour for the crystals to precipitate slowly at room temperature. (The longer you wait, the nicer your crystals will look.) Record your observations. This may take a few minutes, so while you’re waiting, you should work on cleaning up the hot water bath, completing the worksheet, inventorying your glassware, and preparing for the next steps:
- Prepare an ice bath. With the same 800 mL beaker, fill it about halfway with ice and water.
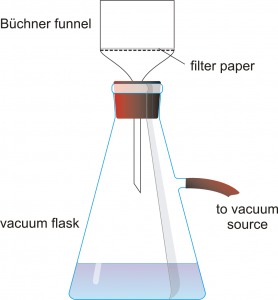 Obtain a Büchner funnel, a filter flask, and an appropriately sized piece of filter paper. Record the mass of the filter paper.
Obtain a Büchner funnel, a filter flask, and an appropriately sized piece of filter paper. Record the mass of the filter paper.- Set up a filter system like the one shown to the left. Wet the filter paper with a few mLs of water so that it sticks to the bottom of the funnel.
- After you have waited about half an hour, put the flask into the ice bath for about ten minutes.
- After that, turn on the vacuum source and pour the crystals and solution into the Büchner funnel. Use a few mLs of ice water to rinse away any crystals that may have stuck to the sides of the flask.
- Turn off the vacuum source once the crystals and paper appear dry.
- Weigh the filter paper and crystals on a tared watch glass. Record the mass on your worksheet and calculate the final mass of crystals.
- Ask your instructor or TA to check your data and observations before you clean up or leave.
Clean up
Pour the filtrate into the waste beaker labeled “lead iodide waste liquid.” Take a picture of your pretty crystals to show your roommate, then put the crystals and filter paper into the waste beaker labeled “solid lead iodide waste.” Pour hot water and ice baths down the sink. Rinse all glassware and return to them to where you found them.
Report
Complete the worksheet. Turn in either a paper or digital copy. This article will help you with the conclusion question. Optional survey.
