The processing of pain involves many neural circuits including those in the spinal cord, brainstem, thalamus, and cortex. When a nociceptive (painful) stimulus is applied, sensory neurons transmit the signal from the skin to the spinal cord. Populations of neurons in the spinal cord further process this signal before transmitting up to the cortex. This is joint work with Victoria Booth and Sofia Piltz, whom I met at a joint AWM and NIMBioS workshop. On this page, I will describe three directions in which we have applied, or are in the process of applying, the spinal cord model to further understand mechanisms underlying pain processing in humans.
Daily rhythmicity of pain sensitivity
Circadian rhythms underlie many biological processes, including the regulation of immune cells that contribute to the processing of pain. As a result, pain sensitivity exhibits a 24-hour rhythm, with the highest sensitivity occurring during the middle of the night and lowest sensitivity occurring in the middle of the afternoon. In neuropathic patients, those who experience chronic pain usually accompanied by damaged nerve tissue, the sensitivity of pain follows a rhythm of opposite phase, with a peak occurring in the mid-afternoon. Unfortunately, mechanisms underlying this shift in rhythmicity for neuropathic pain patients remain unclear, leading to a need for further investigations into circadian modulation of pain processing.
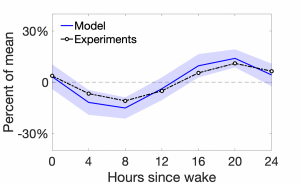
Figure 1: Pain sensitivity as a function of hours since a scheduled wake time. The model results are shown by the blue solid curve, while the experimental results are shown by the dash-dotted black curve.
We introduce circadian influences into our spinal cord model by assuming that the firing rate of the incoming sensory fibers is modulated by the time of the day. We show that, with this assumption, the model reproduces the experimentally-observed daily rhythm of pain; see Figure 1. Then, we propose one possible mechanistic change in circuitry that may explain the flip in rhythmicity for neuropathic patients. Finally, we use the model to investigate how different pain phenomena might be affected by the time of day.
J. Crodelle, M. Hagenauer, S. Piltz, and V. Booth. Modeling the daily rhythm of human pain processing in the dorsal horn. PLOS Computational Biology 15(7): e1007106, (2019).
Sleep and pain have a complex relationship. Rest is necessary for healing and reducing pain, but being in pain inhibits our ability to sleep. I am interested in connecting our spinal cord model of pain processing to a model for sleep dynamics to further understand this interplay between the two essential systems. In particular, I plan to incorporate sleep dynamics by allowing the homeostatic sleep drive to modulate the communication between neuron populations in the spinal cord. In this way, I may use the model to investigate sleep effects on both normal and neuropathic pain.
As a first step in this direction, we used data from a recent experiment measuring changes in pain sensitivity due to sleep deprivation to develop a model connecting sleep and pain sensitivity. We use this model to make predictions about how sleep deprivation due to jet lag or shift work may impact pain sensitivity.
J. Crodelle, C. Vanty*, and V. Booth. Modeling homeostatic and circadian modulation of human pain sensitivity, Frontiers in Neuroscience. 17, 1166203 (2023).
Spinal cord stimulation
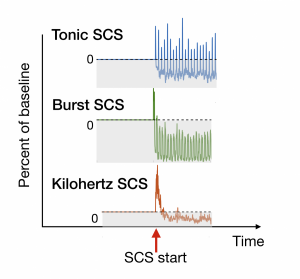
Figure 2: The effect of three different SCS protocols on percent of pain. The gray area indicates a decrease in pain.
Spinal cord stimulation (SCS) is the process by which electrical stimulation is applied to the spinal cord to lessen the effects of chronic pain. There are several proposed mechanisms for how SCS may reduce pain; however, the strength and frequency of stimulation vary per person, and there is no way, as of yet, to determine a priori which method to use. I plan to use the spinal cord model, in conjunction with experiments on SCS, to develop an understanding for how different SCS protocols might affect different spinal-cord circuits. In particular, I plan to incorporate individualized data from clinical patients to make predictions about which SCS protocol might work best for them. Figure 3 shows the effect of three different SCS protocols on one spinal cord circuitry.