Neurons in the developing cortex communicate through gap (or electrotonic) junctions, sites at which neurons directly communicate through the passage of ions and small molecules. As the brain develops, synaptic connections begin to form and largely replace the gap junctions as a means of communication among the excitatory neurons. Gap junctions among the inhibitory neurons remain and mediate signals among them in conjunction with the synaptic connections.
The effect of gap junctions on the orientation-preference map formation
My work in this area focuses on the development of orientation-preference maps in the visual cortex of mice. Some neurons in the primary visual cortex of mammals preferentially respond to stimulus features such as the orientation angle of the edge of a visual stimulus. In higher-level mammals such as monkeys and cats, the visual cortex contains an ordered map of the orientation preference of each neuron where cells that prefer similar angles reside close to one another. In rodents, however, the map of orientation preference appears random and disordered, with little correlation between preferred orientation and location in cortical space. I am interested in modeling the formation of the synaptic connections into and within the visual cortex during development and using this model to reveal mechanisms underlying the creation of different orientation-preference maps across species.
Experiments in mice reveal that neurons are transiently coupled by gap junctions during the first postnatal week, a time at which synaptic connections between neurons are not yet formed, and disappear by the time that orientation preference is determined. In addition, the cells that were coupled by a gap junction during development, have an increased likelihood of forming a similar orientation preference in adulthood, as well as a recurrent synaptic connection. I use integrate-and-fire model neurons to build a model of the visual cortex of mice during the first two postnatal weeks, with Hebbian learning rules to model changes in the synaptic strength among neurons due to their spiking activity. I numerically integrate long-time simulations of this network until the synaptic weights have stabilized, and the neurons have developed an orientation preference.
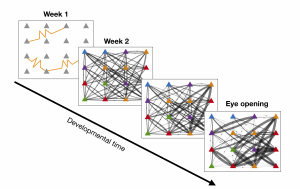
Figure 1: The development of the recurrent cortical synaptic connections for a small subset of the network. The yellow curves during week 1 represent gap junction coupling. The color of each cell represents its orientation preference, and the weight of the line indicated the strength of synaptic coupling.
The model reproduces the experimentally-observed behavior of gap-junction coupled cells such as similarity of orientation preference and formation of synaptic connections; see Figure 1. The model also exhibits a disordered orientation-preference map, as expected. However, we demonstrate that if the recurrent cortical synapses begin to form earlier in developmental time, the resulting orientation-preference map becomes more ordered, until it resembles that of a high-level mammal; see maps in Figure 2. The role of gap-junction coupling in the network seems to be in enhancing disorder in the orientation-preference map.

Figure 2: A measure for the amount of disorder in the orientation-preference map as a function of the time at which recurrent connections are allowed to form (also the time at which gap junctions disappear). The plots shown on either side demonstrate examples of an ordered map (left) and a disordered map (right), where color indicates orientation preference.
J. Crodelle and David W. McLaughlin. Modeling the role of gap junctions between excitatory neurons in the developing visual cortex. PLOS Computational Biology, 17(7):e1007915 (2021). [DOI]
Electrical junctions in the adult cortex
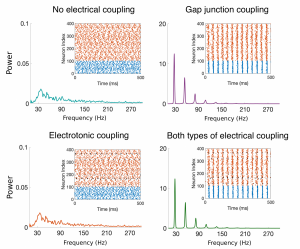
Figure Caption: Raster plots and Power Spectral Density (PSD) plots for four example networks with no electric coupling, just gap-junction coupling among the inhibitory cells, electrotonic coupling among the excitatory cells, and both types of electrical coupling. The broad-band PSD plots for the networks without gap-junction coupling (teal and orange curves) indicate no synchronous oscillations, while the peaks in the PSD plots for those networks containing gap-junction coupling (purple and green curves) indicate synchronized oscillations.
Experimentalists and computational neuroscientists have shown that the presence of gap junctions among inhibitory cells in neuronal networks typically promotes synchronous behavior in the cortex. The role of gap junctions between excitatory cells, however, remains to be determined. My thesis work focused on the adult prefrontal cortex, an area of the brain responsible for higher-level cognitive processes such as learning and attention. I developed a mathematical model including biologically-motivated connectivity probabilities and strengths for both electrotonic junctions (among excitatory cells) and gap junctions (among inhibitory cells), as well as chemical synapses, to investigate the effect of electrical coupling on network synchrony. Through analysis of this model, I showed that gap junctions among inhibitory neurons enhance synchronous oscillations, while the inclusion of electrotonic junctions among the excitatory neurons might play a small role in tightening these synchronized oscillations, but the effect is small and local. Through proposed circuitry changes, the model suggests that the electrical junctions could potentially play a significant role in information processing by enhancing the variability in the firing pattern of the network.
J. Crodelle, D. Zhou, G. Kovačič, D. Cai., A role for electrotonic coupling between cortical pyramidal cells, Frontiers in Computational Neuroscience, 13:33, (2019).