Learning goals: follow instructions to complete a chemistry experiment, collect and analyze experimental data, explain likely sources of experimental error, work collaboratively with a lab partner.
Introduction
Rules about significant figures may seem arbitrary from a theoretical standpoint, but in the laboratory you will see that they allow you to determine the precision of your measurements and calculations. When your measurement has a limited number of digits, your subsequent calculations will also have a limited number of digits.
In this lab, you will be measuring the density of water using a variety of tools. Although your results should be similar every time, the number of significant digits you get will vary depending on the uncertainty associated with the measurement techniques.
Background
Significant digits from common measurements
-
- Mass – analytical balances generally give many significant digits, particularly when weighing 0.1 g or more, you get 4, 5, 6, or 7 significant digits. For example, 0.5012 g of a substance has 4 significant digits. Higher masses give you more significant digits until you reach the capacity of the balance. 319.9999g is 7 significant digits. That is the maximum mass for one of our balances.
- Volume
- Volumetric flasks are extremely precise tools for measuring volume and often give you 4 or more significant digits, depending on the size of the volumetric flask. For example, a precision 1 liter volumetric flask filled exactly to the line etched on the neck contains 1.0000 L, which is 5 significant digits. The precision is usually printed on the flask for reference.
- Micropipettes are very precise tools for measuring extremely small volumes (less than one milliliter). The number of significant digits you get from a micropipette is printed on the pipette for your reference, but it is usually about 4 significant digits.
- Burets are very precise tools for measuring volume. Our lab is equipped with burets that measure to the nearest 0.01 mL, so a volume greater than 1 mL will have 3 significant digits, and a volume greater than 10 mL will have 4 significant digits. You always estimate one more digit than you can read from the lines and estimate to 1/5th between lines.

50 mL graduated cylinder https://images-na.ssl-images-amazon.com/images/I/318fpllZXkL.jpg
- Graduated cylinders are the most flexible tool for measuring volume. Our lab is equipped with many different graduated cylinders, and the number of significant digits they give you depends on the exact graduated cylinder you are using and the volume you are measuring. The number of decimal places you can read is printed on the glass for your reference. Typically you will get 3 significant digits from a graduated cylinder. You always estimate one more digit than you can read from the lines. You will normally estimate to the nearest 1/5th between lines.
- Repeat after me: “Erlenmeyer flasks and beakers are not designed to measure volumes,” although they can be used to get a rough estimate if the volume is not critical. For example, if you are making a hot water bath with approximately 400 mL of water (1 significant digit), then it is appropriate to use a beaker measure that volume.
- Temperature – our red and blue alcohol thermometers are read to the nearest 0.1 °C. This means that we can only read 3 significant digits for temperatures between 10 and 99.9 °C, and we only get 2 significant digits for temperatures between 0 and 9.9 °C. You always estimate one more digit than you can read from the lines. Mercury thermometers give more significant digits, but they have fallen out of favor due to safety concerns when they (inevitably) break.
Significant digits from common calculations
- Adding/subtracting: Use the least number of decimal places involved in the calculation. For example, if you measure a temperature change from 25.0 °C to 28.1 °C, 28.1 – 25.0 = 3.1 °C. See how fast you can lose significant digits in the lab?
- Multiplying/dividing: Use the least number of significant digits involved in the calculation. For example, if you dissolve 0.3829 moles of a substance in 1.0000 L of water, then the concentration is 0.3829 moles per liter. The volume had 5 significant digits, but the number of moles only had 4 significant digits, so you are left with just 4 significant digits in your answer.
- Averaging: We have special rules for averaging multiple measurements. Ideally, if you measure the same thing 3 times, you should get exactly the same result three times, but you usually don’t. The spread of your answers affects the number of significant digits in your average; a bigger spread leads to a less precise average. The last significant digit of the average is the first decimal place in the standard deviation. For example, if your average is 3.025622 and your standard deviation is 0.01845, then this is the correct number of significant figures for the average: 3.03, because the first digit of the standard deviation is in the hundredths place, so the last significant digit of the average is in the hundredths place.
Rules for rounding
Numbers between 6 and 9 round up.
Numbers between 1 and 4 round down.
5s round up or down to an even number. For example, if your average in lab is 92.5, the 5 would round down to 92, so an A- letter grade. If your average is 89.5, the 5 would round up to 90, so an A- letter grade.
Procedure
1. Measure the density of 5 mL of water with a graduated cylinder
Use an analytical balance to measure the mass of an empty, dry 50 mL beaker or Erlenmeyer flask. Remember to zero the balance before you use it. Record the mass in Table 1.
Measure out 5 mL of DI water with a 50-mL graduated cylinder. Read the exact volume at the bottom of the meniscus and record it in Table 1. Be sure to record all significant figures. Estimate to the nearest 1/5th mL (1/5th between lines). You will estimate the last decimal place.
Transfer the contents of the graduated cylinder to the beaker or Erlenmeyer flask. Use an analytical balance to measure the mass of the beaker that now contains 5 mL of water. Remember to zero the balance before you use it. Record the mass in Table 1.
2. Measure the density of 50 mL of water with a graduated cylinder
Use an analytical balance to measure the mass of an empty, dry 50 mL beaker or Erlenmeyer flask. Remember to zero the balance before you use it. Record the mass in Table 1.
Measure out 50 mL of DI water with a 50-mL graduated cylinder. Read the exact volume at the bottom of the meniscus and record it in Table 1. Be sure to record all significant figures. Estimate to the nearest 1/5th mL (1/5th between lines). You will estimate the last decimal place.
Transfer the contents of the graduated cylinder to the beaker or Erlenmeyer flask. Use an analytical balance to measure the mass of the beaker that now contains 50 mL of water. Remember to zero the balance before you use it. Record the mass in Table 1.
3. Measure the density of water with a volumetric flask
Use an analytical balance to measure the mass of an empty, clean, dry 100 mL beaker or Erlenmeyer flask. Remember to zero the balance before you use it. Record the mass in Table 1.
Fill a 100 mL volumetric flask with DI water until the bottom of the meniscus is exactly at the etched line. You may need to use a dropper to reach the line exactly. Record the volume to the tenth place: 100.0 mL. Record that volume in Table 1.
Transfer the contents of the volumetric flask to the beaker or Erlenmeyer flask. Use an analytical balance to measure the mass of the beaker that now contains 100 mL of water. Note that not all of the balances in the lab may be able to measure this mass. Use a different balance if you get an error. Remember to zero the balance before you use it. Record the mass in Table 1.
Data analysis
Review the rules about significant figures, then calculate the mass of water, volume of water, and density of water for each method. Fill in Table 2. Note: density = mass ÷ volume.
Report
Fill out this worksheet. Turn in either a paper or digital copy. You may use this table to look up the correct density of water at a variety of temperatures.



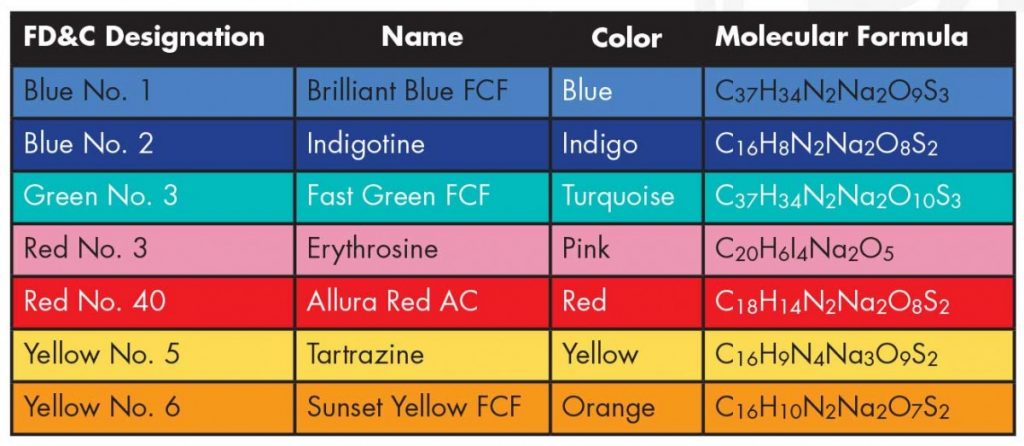



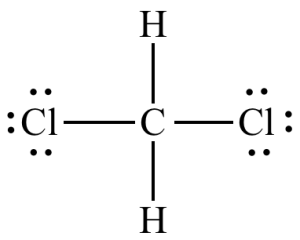

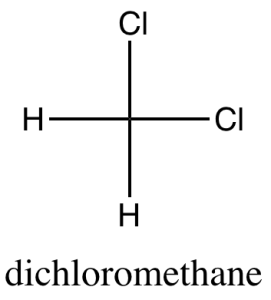 software. It calculates 3D structures and molecular orbitals, which help chemists predict the chemical and physical properties of theoretical molecules. In this lab, you will use ChemDraw to analyze the structures of molecules in 3D.
software. It calculates 3D structures and molecular orbitals, which help chemists predict the chemical and physical properties of theoretical molecules. In this lab, you will use ChemDraw to analyze the structures of molecules in 3D.

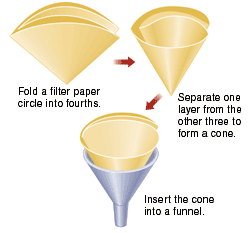

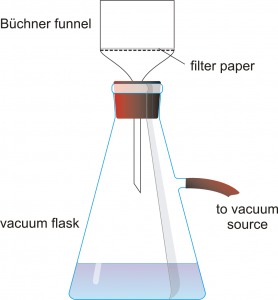 Obtain a Büchner funnel, a filter flask, and an appropriately sized piece of filter paper. Record the mass of the filter paper.
Obtain a Büchner funnel, a filter flask, and an appropriately sized piece of filter paper. Record the mass of the filter paper.